Anthocyanidins
es
fr
pt

Anthocyanins & Anthocyanidins
As one of the leading manufacturers internationally, PhytoLab offers over 1,400 extensively documented herbal reference substances of all classes of natural compounds. Our portfolio currently includes a total of 21 anthocyanins and 7 anthocyanidins. Most of our anthocyanins and all our anthocyanidins are certified as primary reference standards.
Anthocyanins are water-soluble secondary plant metabolites that can occur in all parts of higher plants, including leaves, stems, roots, flowers and fruits. The name derives from the Greek: words ἀνθός (anthos) = flower and κυανός (kyanos) = blue. Depending on pH-value, anthocyanins may also appear orange, red and purple. In nature, anthocyanins are responsible for making bright-colored flowers and fruits attractive to pollinators or animals. Anthocyanins also act as a “sunscreen” and protect cells from damage due to exposure to UV-light. They may also act as antioxidants in the cell vacuoles. The antioxidant properties have been confirmed in vitro, although this effect is most likely limited in vivo due to low bioavailability of the intact anthocyanins. However, anthocyanin metabolites may have an indirect effect.
Structure of common anthocyanidins
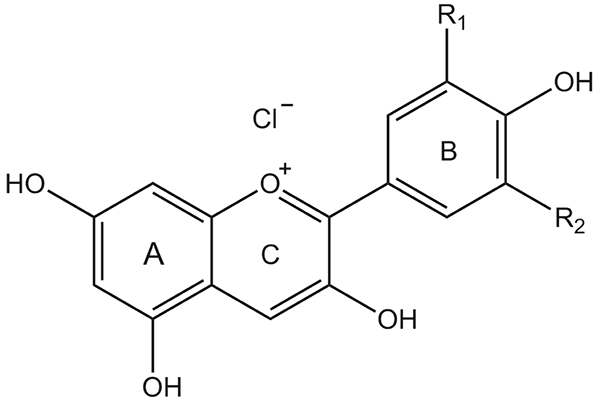
Anthocyanins belong to the class of natural compounds known as flavonoids. Their 15-carbon skeleton consists of two phenyl rings and one heterocyclic ring containing a positively charged oxygen atom. In nature, usually carboxylate anions of water-soluble acids would act as counter ions, while the pure compounds are most frequently isolated as chloride salts. The most common anthocyanins exhibit hydroxyl functions in positions 3, 5, 7 and 4’. The anthocyanidins are the aglycones of the anthocyanins, which most often bear a sugar moiety bound to position 3. Structural variation is usually achieved by the substitution pattern in the B-ring and differences in the glycosidic profile.
| Anthocyanidin | R1 | R2 |
|---|---|---|
| Cyanidin | OH | H |
| Delphinidin | OH | OH |
| Malvidin | OCH3 | OCH3 |
| Pelargonidin | H | H |
| Peonidin | OCH3 | H |
| Petunidin | OH | OCH3 |
Occurrence
Selection of anthocyanin-rich plants and compounds contained therein:
* Hybrid grapes also contain anthocyanin diglucosides such as cyanidin 3,5-diglucoside, delphinidin 3,5-diglucoside, malvidin 3,5-diglucoside and peonidin 3,5-diglucoside
Some less known anthocyanin-containing varieties of common foodstuffs exist, e.g. purple potatoes (Solanum tuberosum), purple corn (Zea mays) or purple carrots (Daucus carota).
Pharmacopoeial information
In European Pharmacopoeia, a specification for total content of anthocyanins, calculated as cyanidin 3-glucoside, is given in the monographs for fresh bilberry fruit and fresh bilberrry fruit dry extract, refined and standardised. The latter monograph also specifies a maximum limit for anthocyanidins, calculated as cyanidin, and requests a certain chromatographic profile to confirm identity. While a photometric assay for anthocyanins is used in the monograph for fresh bilberry fruit, an HPLC method identifying 15 anthocyanins is used in the dry extract monograph. Among these 15 anthocyanins PhytoLab is offering delphinidin 3-galactoside, delphinidin 3-glucoside, cyanidin-3 galactoside, cyanidin 3-glucoside, cyanidin 3-arabinoside, petunidin 3-glucoside, peonidin 3-glucoside, malvidin 3-galactoside and malvidin 3-glucoside as comprehensively characterized reference standards. For the determination of anthocyanidins, the peaks due to cyanidin, delphinidin, malvidin, peonidin and petunidin are considered. All these items are available as primary reference substances.
A minimum content of procyanidins, expressed as cyanidin, is given in the European Pharmacopoeia monograph on hawthorn berries.
In United States Pharmacopoeia, the dietary supplements monograph on powdered bilberry extract specifies a minimum content of anthocyanins, calculated as cyanidin 3-glucoside, and a maximum limit for anthocyanidins, calculated as cyanidin. Requirements on chromatographic profiles including peak intensities of various anthocyanins are given. delphinidin 3-galactoside and delphinidin 3-glucoside are described as the most intense peaks, both being more intense than the peak due to cyanidin 3-glucoside, which is of similar intensity than the peaks caused by cyanidin-3 galactoside and delphinidin 3-arabinoside. A total of 15 anthocyanins are described in the HPLC chromatogram, among these we are offering delphinidin 3-galactoside, delphinidin 3-glucoside, cyanidin-3 galactoside, cyanidin 3-glucoside, cyanidin 3-arabinoside, petunidin 3-glucoside, peonidin 3-glucoside, malvidin 3-galactoside and malvidin 3-glucoside. Most of these anthocyanins are primary reference substances. For the determination of anthocyanidins, the peaks due to cyanidin, delphinidin, malvidin, peonidin and petunidin are considered.
The United States Pharmacopoeia monograph on European elder berry dry extract specifies a minimum content of anthocyanins, calculated as the sum of the chloride salts of cyanidin 3-sambubioside 5-glucoside, cyanidin 3,5-diglucoside, cyanidin 3-sambubioside and cyanidin 3-glucoside on the anhydrous basis. A maximum limit for anthocyanidins, calculated as anhydrous cyanidin chloride, is given as well. Requirements on chromatographic profiles including peak intensities of various anthocyanins are given. The most intense peak is due to cyanidin 3-sambubioside, followed by cyanidin 3-glucoside. The peak due to cyanidin 3-sambubioside 5-glucoside is substantially smaller. cyanidin 3,5-diglucoside and cyanidin represent minor peaks only.
Recommendation
For a reliable quantitative analysis of anthocyanins & anthocyanidins well characterized reference substances are essential. Currently we offer 28 anthocyanins & anthocyanidins, all of them supplied together with a comprehensive certificate of analysis. Due to the positive charge of the molecule the counter ion has to be taken into account. For all anthocyanins & anthocyanidins characterized as primary reference substances, chloride (or perchlorate in case of the dracorhodin) was determined quantitatively and considered as an impurity in the calculation of the absolute content, which therefore refers to the pure anthocyanin or anthocyanidin only.
For up-to-date information on prices and specifications please check the product detail pages below!
Reference Substances
- Cyanidin 3-arabinoside chloride
- Cyanidin chloride
- Cyanidin 3,5-diglucoside chloride
- Cyanidin 3-galactoside chloride
- Cyanidin 3-glucoside chloride
- Cyanidin 3-(6''-malonylglucoside)
- Cyanidin 3-rutinoside chloride
- Cyanidin 3-sambubioside chloride
- Cyanidin 3-sophoroside chloride
- Delphinidin chloride
- Delphinidin 3,5-diglucoside chloride
- Delphinidin 3-galactoside chloride
- Delphinidin 3-glucoside chloride
- Delphinidin 3-rutinoside chloride
- Delphinidin 3-sambubioside chloride
- Dracorhodin perchlorate
- Malvidin chloride
- Malvidin 3,5-diglucoside chloride
- Malvidin 3-galactoside chloride
- Malvidin 3-glucoside chloride
- Pelargonidin chloride
- Pelargonidin 3,5-diglucoside chloride
- Pelargonidin 3-glucoside chloride
- Peonidin chloride
- Peonidin 3,5-diglucoside chloride
- Peonidin 3-glucoside chloride
- Petunidin chloride
- Petunidin 3-glucoside chloride


