As one of the leading manufacturers internationally, PhytoLab offers over 1,500 extensively documented herbal reference substances of all classes of natural compounds. Our portfolio currently includes a total of 28 glucosinolates as well as the goitrogenic degradation product DL-Goitrin. Most of our glucosinolates and related compounds are certified as primary reference standards.

Glucosinolates
Occurrence and properties
Glucosinolates are secondary plant metabolites that occur in a wide variety of plants mainly from the families of the Brassicaceae (e.g. horseradish (Armoracia rusticana), radish (Raphanus sativus), wasabi (Eutrema japonicum), black mustard (Brassica nigra), white mustard (Sinapis alba), broccoli (Brassica oleracea var. italica), rapeseed (Brassica napus), maca (Lepidium meyenii), crambe (Crambe abyssinica), stonecrop (Alyssum argenteum), false flax (Camelina sativa), winter cress (Barbarea vulgaris), water cress (Nasturtium officinale), hoary alyssum (Berteroa incana), wallflower (Cheiranthus cheiri), rocket (Eruca sativa), dame’s violet (Hesperis matronalis), bitter candytuft (Iberis amara) and woad (Isatis tinctoria)), the Capparaceae (e.g. capers (Capparis spinosa)) and the Caricaceae (e.g. papaya (Carica papaya)), but also from the Euphorbiaceae, the Tropaeolaceae (e.g. garden nasturtium (Tropaeolum majus)), the Cleomaceae (e.g. spiny spider flower (Cleome spinosa)), the Limnanthaceae (e.g. Douglas’ meadowfoam (Limnanthes douglasii)) and the Moringaceae (e.g. drumstick tree (Moringa oleifera)).
Besides being responsible for the pungent and bitter taste of these plants, the glucosinolates and their hydrolysis products also protect plants against herbivores and have been shown to have antimicrobial, antiviral, antifungal and anticarcinogenic properties. Due to their antimicrobial properties, herbal medicinal products containing nasturtium herb and horseradish root are used in the treatment of sinusitis, bronchitis and urinary tract infections. The German Commission E published a monograph on horseradish (Armoraciae rusticanae radix) in 1988.
Structure of glucosinolates and derived compounds

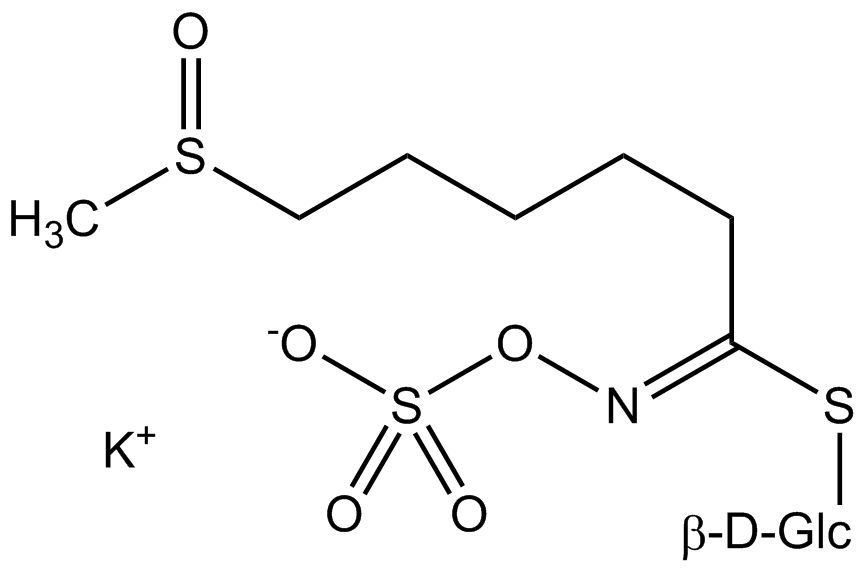
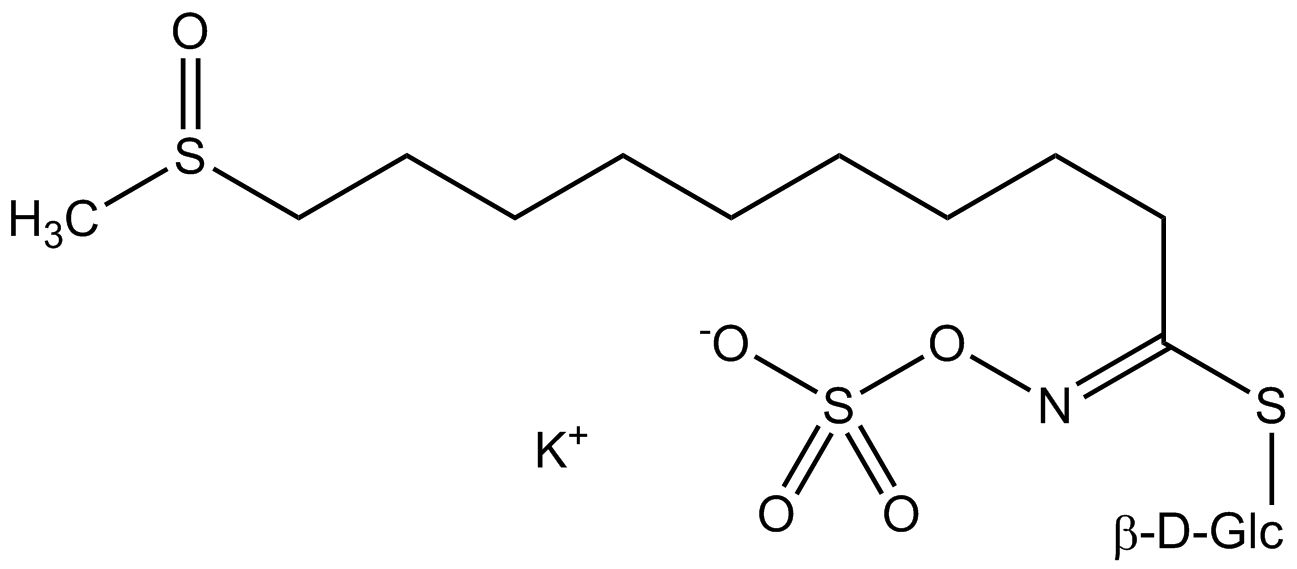
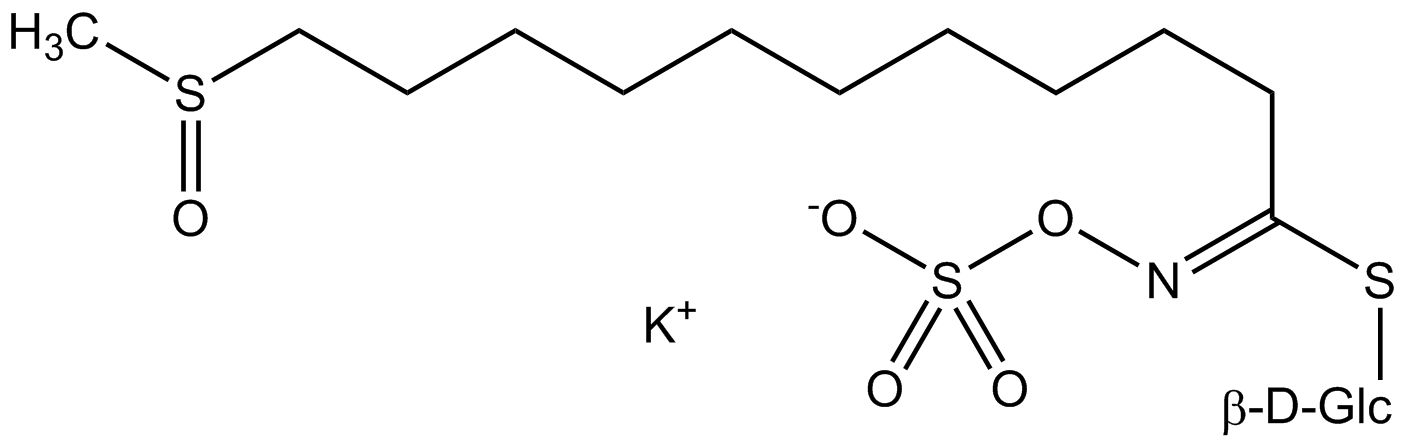
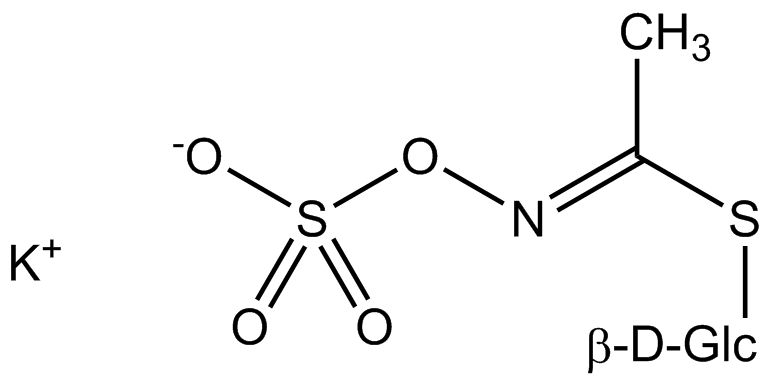
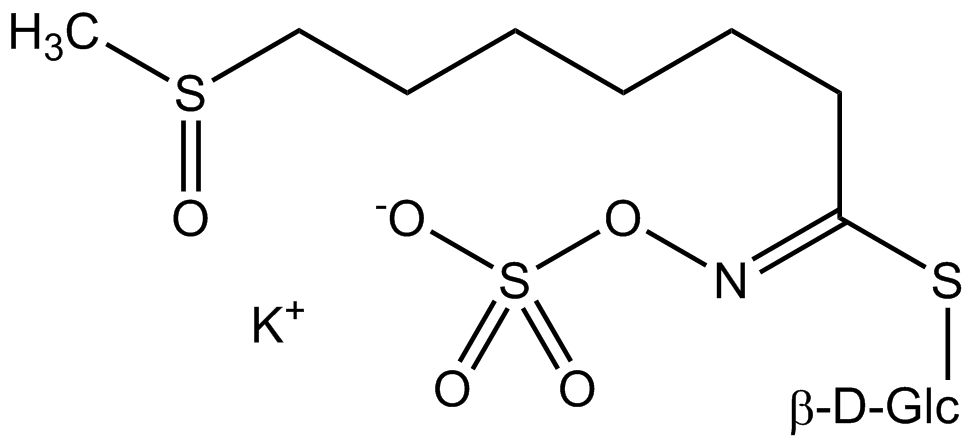
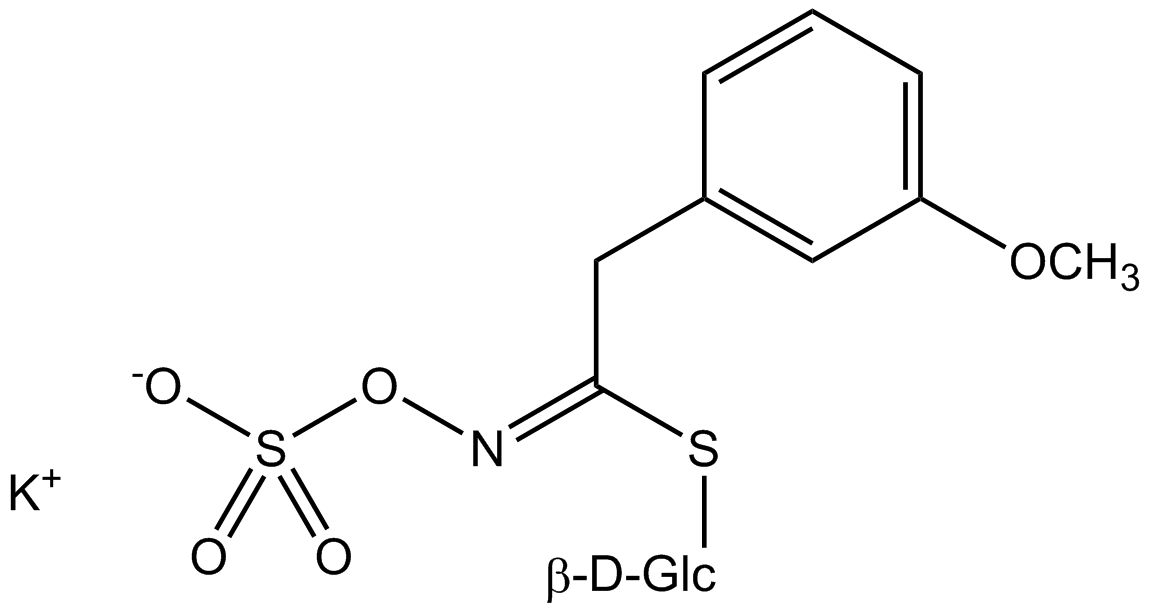
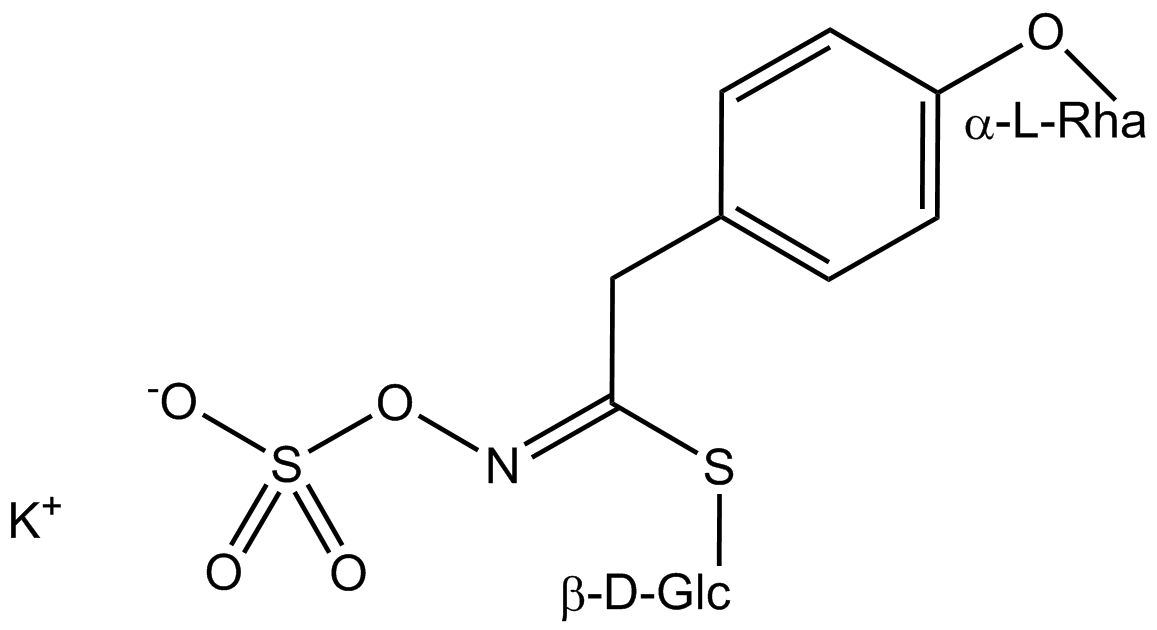
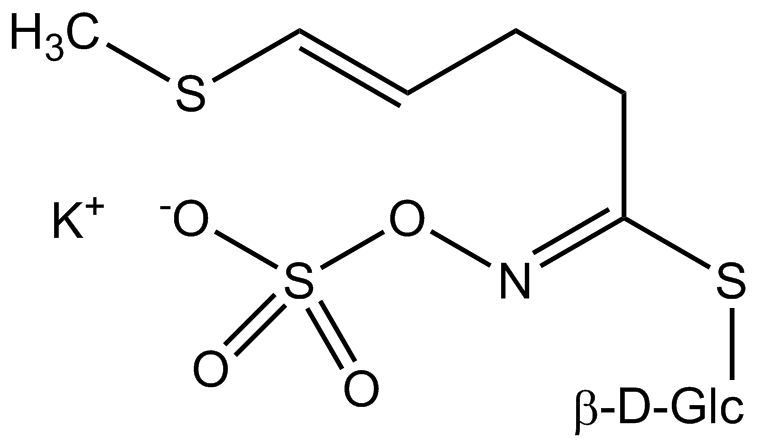
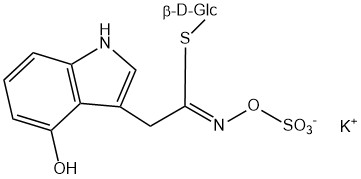
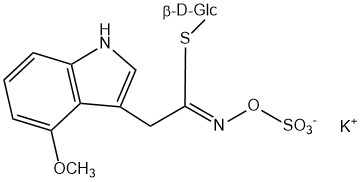
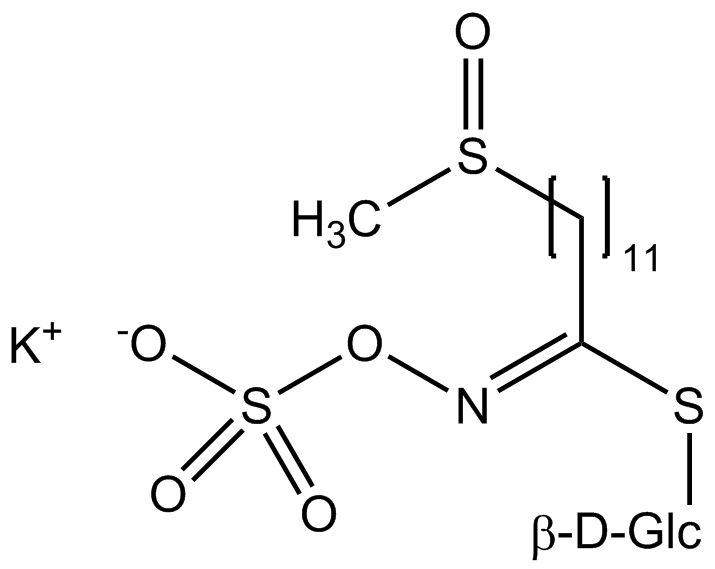
All glucosinolates are composed of a central carbon that is bound via a sulfur atom to a glucose, and via a nitrogen atom to a sulfate group. Furthermore, a substance-specific side chain (its structure depending on the amino acid involved in the initial phase of the glucosinolate biosynthesis) is bound to the central carbon atom. As the sulfate group is negatively charged, glucosinolates are most often isolated in the form of their potassium salts. Biosynthesis of glucosinolates that bear an alkyl or a sulfur-containing side chain (such as thiomethyl-, sulfinyl- or sulfonyl groups) starts most frequently from methionine (such as epiprogoitrin, glucoalyssin, glucoarabin, glucoberteroin, glucobrassicanapin, glucocamelinin, glucocapparin, glucocheirolin, glucoerucin, glucohesperin, glucoiberin, gluconapin, glucoraphanin, glucoraphasatin, glucoraphenin, 11-methylsulfinylundecylglucosinolate, progoitrin and sinigrin). The biosynthesis of glucosinolates with a phenyl or benzyl ring in the core structure starts from either phenylalanine or tyrosine (e.g. glucobarbarin, glucolimnanthin, glucomoringin, gluconasturtiin, glucotropaeolin and sinalbin), while indolyl-containing side chains as in glucobrassicin, 4-Hydroxyglucobrassicin, 4-Methoxyglucobrassicin and Neoglucobrassicin originate from the amino acid tryptophane.
Upon contact with the enzyme myrosinase and water (myrosinase is kept in a separate compartment in the cell, but can be released e.g. during cutting or chewing), the glucose moiety is cleaved. The remaining molecule can then undergo various spontaneous reactions, usually resulting in the corresponding isothiocyanate. Depending on the reaction conditions also thiocyanates, nitriles or oxazolidine 2-thiones such as the goitrin can be formed.

Glucosinolates: side chain structure and derived isothiocyanates
| Glucosinolate | Side chain R | Corresponding isothiocyanate |
|---|---|---|
| Epiprogoitrin | CH2=CH–CHOH–CH2– | 2(S)-Hydroxy 3-butenylisothiocyanate |
| Glucoalyssin | CH3–SO–(CH2)5- | 5-(Methylsulfinyl)pentylisothiocyanate |
| Glucoarabin | CH3–SO–(CH2)9- | 9-(Methylsulfinyl)nonylisothiocyanate |
| Glucobarbarin | C6H5–CHOH–CH2– | 2(S)-Hydroxy 2-phenylethylisothiocyanate |
| Glucoberteroin | CH3–S–(CH2)5– | 5-(Methylthio)pentylisothiocyanate |
| Glucobrassicanapin | CH2=CH2–(CH2)3– | 4-Pentenylisothiocyanate |
| Glucobrassicin | 3-Indolylmethyl- | 3-Indolylmethylisothiocyanate |
| Glucocamelinin | CH3–SO–(CH2)10- | 10-(Methylsulfinyl)decylisothiocyanate |
| Glucocapparin | CH3– | Methylisothiocyanate |
| Glucocheirolin | CH3–SO2–(CH2)3– | 3-(Methylsulfonyl)propylisothiocyanate |
| Glucoerucin | CH3–S–(CH2)4– | 4-(Methylthio)butylisothiocyanate |
| Glucohesperin | CH3–SO–(CH2)6- | 6-(Methylsulfinyl)hexylisothiocyanate |
| Glucoiberin | CH3–SO–(CH2)3– | 3-(Methylsulfinyl)propylisothiocyanate |
| Glucolimnanthin | OCH3-C6H5–CH2– | 3-Methoxybenzylisothiocyanate |
| Glucomoringin | 4-O-Rhamnosy-C6H4–CH2– | 4-(Rhamnosyloxy)benzylisothiocyanate |
| Gluconapin | CH=CH–(CH2)2– | 3-Butenylisothiocyanate |
| Gluconasturtiin | C6H5–(CH2)2– | 2-Phenylethylisothiocyanate |
| Glucoraphanin | CH3–SO–(CH2)4– | Sulforaphane |
| Glucoraphasatin | CH3–S–CH=CH–(CH2)2– | 4-(Methylthio)-3-butenylisothiocyanate |
| Glucoraphenin | CH3–SO–CH=CH–(CH2)2– | Sulforaphene |
| Glucotropaeolin | C6H5–CH2– | Benzylisothiocyanate |
| 4-Hydroxyglucobrassicin | 4-Hydroxy-3-indolylmethyl | 4-Hydroxy-3-indolylmethyl isothiocyanate |
| 4-Methoxyglucobrassicin | 4-Methoxy-3-indolylmethyl | 4-Methoxy-3-indolylmethyl isothiocyanate |
| 11-Methylsulfinylundecyl-glucosinolate | CH3–SO–(CH2)11- | 11-(Methylsulfinyl)undecylisothiocyanate |
| Neoglucobrassicin | N-Methoxy-3-indolylmethyl | N-Methoxy-3-indolylmethyl isothiocyanate |
| Progoitrin | CH2=CH–CHOH–CH2– | 2(R)-Hydroxy-3-butenylisothiocyanate |
| Sinalbin | 4-OH-C6H4–CH2– | 4-Hydroxybenzylisothiocyanate |
| Sinigrin | CH2=CH–CH2– | Allylisothiocyanate |
Recommendation
For a reliable quantitative analysis of glucosinolates and their degradation products well characterized reference substances are essential. Currently we offer 28 glucosinolates as well as the goitrogenic degradation product DL-Goitrogen, all of them supplied together with a comprehensive certificate of analysis. Due to the negative charge of the glucosinolate core structure the counter ion has to be taken into account. For all glucosinolates characterized as primary reference standard, potassium was determined quantitatively and considered as an impurity in the calculation of the absolute content, which therefore refers to the pure glucosinolate only.
For up-to-date information on prices and specifications please check the product detail pages below!